Iatrogenesis and the Poisonous Nature of Fluoroquinolone Antibiotic Drugs Such as Cipro
Definition: An iatrogenic disease is an illness that occurs as a result of a therapeutic or diagnostic procedure undertaken on a patient; a healthcare professional-caused disease, usually due to properly-prescribed prescription drugs, vaccines or surgical procedures.
The following information concerns the serious toxic effects of fluoroquinolone antibiotics - which include Bayer’s Cipro, Janssen’s Levaquin, Bayer’s Avelox, Merck’s Noroxin, Pfizer’s Trovan and the generic drug Ofloxacin.
The information outlined below is excerpted from three sources that I have only become aware of recently.
1) a 29-page FDA document that discusses fluoroquinolone antibiotic-caused peripheral neuropathy.
The document can be found in its entirety at https://drive.google.com/file/d/0BzLMHZg5q0Y3VkVJUmhxSlQtbWs/edit.
This document totally ignores the equally serious poisonous effects of fluoroquinolone drugs such as the antibiotic’s toxic effects on cellular mitochondria, which is the likely cause of the tendonopathies, neuropsychiatric disorders, chronic fatigue syndromes, muscular disorders, cardiomyopathies, cardiac dysrhythmias, neurodegenerative disorders, etc
2) Some of the information has been excerpted from http://www.saferpills.org/wp-content/uploads/2014/08/Citizen-Petition-from-the-Southern-Network-on-Adverse_Reactions.pdf.
3) I also attach a relevant abstract from a 2001 British Medical Journal article about Pfizer’s malfeasance in its testing of ts fluoroquinolone drug (Trovan) during a 1996 Nigerian meningitis epidemic.
I feel that such information about once popular prescription drugs - that have been deceptively advertised by Big Pharma as safe - is particularly important because I have been among the multitude of healthcare providers that were intentionally deceived by Big Pharma into believing their false claims of safety for any number of now-known to be dangerous vaccines, psych drugs, arthritis drugs, heart drugs, etc.
This article is about one of the classes of antibiotics that have now been revealed to be commonly toxic. Therefore I feel it is my duty to warn readers that there will surely be more heavily propagandized, synthetic drug products from Big Pharma that will be far less safe than will be claimed. It is also important for patients to be aware that the adverse effects they experienced weren’t psychosomatic or coincidences of nature. Knowing the root causes of illnesses makes for more rational treatments approaches.
I had heard in the past about patients that had occasionally developed Cipro-related peripheral neuritis, tendonitis and even tendon ruptures but only recently have I become aware of the mechanisms of action for the toxicity. The drug companies that manufactured Cipro et al never alerted me about how common were the drug’s dangers, and thus I never had the chance to warn any of my patients about those dangers.
Therefore I was partially guilty, albeit inadvertently - of causing iatrogenic diseases. Fortunately, to my knowledge, none of my patients ever died because of my prescribing the drug, but I would be willing to bet that some of them were significantly sickened – perhaps permanently. Iatrogenic diseases are usually caused by the supposedly appropriate prescribing of drugs and vaccines. Iatrogenic diseases are actually the third leading cause of death in the US, close behind heart disease and cancer. Many iatrogenic diseases are naturally under-reported by physicians on hospital discharge notes and by coroners on death certificates, so we will probably never know the true statistics.
One wonders how many iatrogenic diseases are non-lethal. Again there is the problem of under-reporting. To estimate the seriousness of the problem, one only has to consider the story of the 50,000+ cardiac deaths that were caused by Merck’s arthritis drug Vioxx before it was withdrawn from the market. One also has to wonder how many patient’s hearts were sub-lethally poisoned by Vioxx and by the two similar COX-2 inhibitor arthritis drugs Celebrex and Bextra, which are apparently still on the market. And one has to wonder if those drugs were mitochondrial toxins, a reality that is now known to be the case for many vaccines that have had mercury or aluminum in it.
Read this and then start being more suspicious of the propaganda coming out of the public relations departments of Big Pharma.
The FDA Adverse Event Reporting System (FAERS) data summarized below represents a compilation of14 years of complaints against the 3 best-selling fluoroquinolones antibiotics; Janssen’s Levaquin (79,328 complaints), Bayer’s Cipro (67,498 complaints), and Bayer’s Avelox (57,821 complaints). (It is worth recalling that the FDA acknowledges that they only receive 1 - 10% of actual complaints, so these complaints could be multiplied by a figure of 100.)
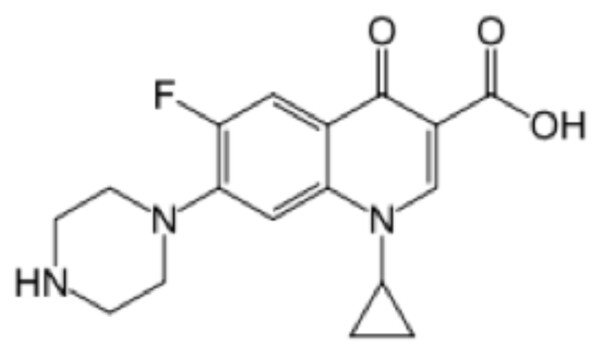
This is the molecular structure of Bayer’s Cipro (note the single fluoride atom, making the drug long-acting but also more cytotoxic and less metabolizable)
Here is a list of the 6 fluoride-containing fluoroquinolone antibiotics that haven’t been banned yet: (Note that both Trovan/Trovafloxacin [Pfizer - dod: 2001] and Gatifloxacin (Bristol-Myers Squibb - dod: 2006] were withdrawn from the market due to well-publicized, very serious Mitochondria Toxicity, which is a trait of ALL fluoroquinolones drugs, including the ones still on the market.)
Noroxin® (norfloxacin)—Merck and Co. Cipro® Cipro XR® (ciprofloxacin)—Bayer HealthCare Levaquin® (levofloxacin)—Janssen (subsidiary of J & J) Pharmaceuticals Avelox® (moxifloxacin)—Bayer HealthCare Factive® (gemifloxacin)—Cornerstone Therapeutics Ofloxacin—generic

Here is the molecular structure of the banned Trovafloxacin from Pfizer (note the THREE fluoride atoms, making the molecule much longer acting, much more likely to irreversibly bind to other molecules [including to brain tissue, peripheral nerves, tendons, enzymes and DNA – both bacterial and mammalian!] and also much more likely to be poisonous [hence its removal from the market in 2001]
“Fluoroquinolones may cause Mitochondrial Toxicity due, in part, to an insufficiency of ATP. Mitochondrial conditions that are due to an insufficiency of ATP include developmental disorders of the brain, optic neuropathy, neuropathic pain, hearing loss, muscle weakness, cardiomyopathy, and lactic acidosis. Neurodegenerative diseases, like Parkinson's, Alzheimer's and amyotrophic lateral sclerosis (ALS) have been associated with the loss of neurons due to oxidative stress generated by reactive oxygen species (ROS) related to Mitochondrial Toxicity. Peripheral neuropathy, hepatoxicity, glucose disturbances, and phototoxicity may result from Mitochondrial Toxicity.”
Below is a citizen’s request to Johnson and Johnson (makers of Levaquin) suggested that a black box warning be attached to future Levaquin product information inserts. According to Wikipedia (heavily influenced/controlled by industry), as of 2017, the FDA has not complied with the request concerning adding a Black Box warning about mitochondrial toxicity. (!!) Tellingly, WikiPedia never mentions mitochondrial toxicity.
Here was the black box suggestion: “Fluoroquinolones may cause Mitochondrial Toxicity. Mitochondrial Toxicity has been implicated in conditions such as peripheral neuropathy, hepatoxicity, glucose disturbances, phototoxicity, developmental disorders of the brain, optic neuropathy, neuropathic pain, hearing loss, muscle weakness, cardiomyopathy, lactic acidosis, Parkinson's, Alzheimer's, and amyotrophic lateral sclerosis (ALS).” BMJ 2001 Jan 27; 322(7280): 194.
Pfizer accused of testing new antibiotic (on Nigerian children) without ethical approval
By Jacqui Wise https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1119465/ An official inquiry has been set up into allegations that the drug manufacturer Pfizer did not obtain official approval before testing a new drug on children during a meningitis epidemic in Nigeria five years ago. The Nigerian doctor who supervised the clinical trial has said that his office backdated an approval letter and this may have been written a year after the study had taken place. Pfizer, whose headquarters are in New York city, has admitted that the local ethics approval given to conduct the trial may not have been properly documented: “Pfizer takes this issue very seriously and is fully cooperating with the Nigerian authorities.” In 1996 Pfizer sent a team to Kano in the north of Nigeria during an epidemic of meningococcal meningitis. To test the efficacy of its new antibiotic trovafloxacin (Trovan) they carried out an open label trial in 200 children, half of whom were given trovafloxacin and half the gold standard treatment for meningitis, ceftriaxone. Five of the children given trovafloxacin died, together with six who were given ceftriaxone. Pfizer said that 15000 people died during the epidemic. The Washington Post has been investigating the trial and alleges that at least one child was not taken off the experimental drug and given the standard drug when it was clear that her condition was not improving—which is against ethical guidelines. The newspaper also claims that Nigerian patients were not warned that animal studies had shown that drugs similar to trovafloxacin may cause joint damage, whereas US patients were told of the research in a subsequent trovafloxacin trial. The drug's license was withdrawn in Europe because of liver toxicity and some deaths.
Charles Medawar, director of Social Audit, the UK pressure group that monitors the pharmaceutical industry, said: “This particular case looks to be very bad, but I hardly think it is untypical.”
Of the FDA Adverse Event Recording System (FAERS) complaints against just Levaquin, here is a breakdown of the 9 most common drug-related symptoms that were likely mitochondrial toxicity-related. The top five (ie, most common) complaints were related to tendon toxicity issues and apparently neuropathy symptoms. The 9 less common complaints were these:
Dyspnea 4%, Insomnia (3,173 complaints = 4%), Myalgia (muscle pain) 4%, Pain (2,380 = 4%), Dizziness 3%, Nausea 3%, Gait disturbance 3%, Asthenia Generalized weakness) 3%, Pyrexia 3%. “Peripheral neuropathy is an identified risk with the fluoroquinolones. It was added to the Warnings or Warnings and Precautions sections of all of the fluoroquinolone labels in 2004.
“Initial, broad searches of the Adverse Event Reporting System (AERS) database identified over 1000 reports (see Appendix Table 8.7). In order to find serious cases of prolonged, disabling neuropathy, much narrower search criteria were used, including an outcome of disability. Presumably, these disability cases are among the most severe of all the reported cases. In this case series, 80% of the patients had not recovered from their peripheral neuropathy and the symptoms were still on-going at the time the report was submitted. Only 10% reported improvement or recovery. In addition, the duration of peripheral neuropathy ranged from 1 day to 7 years, with 40% having symptoms for a year or longer, again, depending on when the report was submitted.
“It may be hard to determine at what point an adverse event is considered permanent, but since the outcome for these reports was given as disability, it is reasonable that the reporter considered it to be a permanent condition. Some narratives described circumstances where a healthy, young, athletic patient took a fluoroquinolone for sinusitis or a urinary tract infection and subsequently was unable to run or ambulate without use of a cane. In a few cases, disability was so severe that continued employment was not possible.
“Overall, this review did not identify any predictable risk factors for peripheral neuropathy. The rapid onset of peripheral neuropathy after beginning a fluoroquinolone is an important finding in this review. Symptom onset seemed to be unrelated to the duration of therapy.
The median onset was 4 days and 62% had a symptom onset within 5 days; some patients had symptoms after 1 dose. Duration of drug therapy also did not appear to be a factor (34% were on the drug for 5 days or less and only 16% for more than 14 days). Only 24% of patients had documented risk factors, none had renal dysfunction, and, age was not a significant factor (32% were 40 years of age or less, 14% were 65 years of age or older).
“even patients stated that when they reported peripheral neuropathy to their physician, they were told to continue taking the fluoroquinolone. Some of these patients were told that this class of drugs could not cause peripheral neuropathy.
“When the reporting year was examined, 3 occurred before peripheral neuropathy was added to the Warnings and Precautions section of the labels (July 2004), and 4 were after that time. The agency still receives reports for this adverse event.
“The findings in this review are very similar to those found in the 2003 review. That review did not limit the search to an outcome of disability, but it also found that most patients were relatively young, healthy, and had no conditions predisposing them to peripheral neuropathy. In addition, the onset was rapid (within a few days), there was rapid progression, and the neuropathy could be irreversible. None of those 108 cases (from the 2003 review) reported a complete recovery.
CONCLUSION
“In conclusion, FDA continues to receive reports of fluoroquinolones and peripheral neuropathy, an identified risk of fluoroquinolone antibiotic use. The peripheral neuropathy in many cases appears to be unresolved with 40% having symptoms for a year or longer. The onset of peripheral neuropathy after starting fluoroquinolone therapy is rapid. This review did not identify a relationship between peripheral neuropathy and the duration of therapy, dose of the drug, or the age of the patient, and no specific risk factors were identified. “The current fluoroquinolone labels are inconsistent in the details regarding the risk of peripheral neuropathy and do not describe the possible permanence of peripheral neuropathy, rapid onset, nor the need to consider discontinuation of drug with first symptoms.”
